July 2 2019 Approval Letter - FluLaval. See full prescribing information for FLULAVAL QUADRIVALENT.
Https Www Izsummitpartners Org Content Uploads 2017 05 12b 2 Rodriguez Maternal Vax Communication Cdc Update Pdf
Public Health Service USPHS requirements for the 2020-2021 influenza season and is formulated to contain 60 micrograms mcg hemagglutinin HA per 05mL dose in the recommended ratio of 15 mcg HA of each of the following 4 influenza virus strains 2 A strains and 2 B strains.
Flulaval package insert. QUALITATIVE AND QUANTITATIVE COMPOSITION FluLavalŽ is an inactivated influenza vaccine split virion containing antigens propagated in. FluLaval Quadrivalent is a vaccine indicated for active immunization for the prevention of disease caused by influenza A subtype viruses and type B viruses contained in the vaccine. February 6 2020 Approval Letter - FluLaval.
FLULAVAL QUADRIVALENT is a vaccine indicated for active immunization for the prevention of disease caused by influenza A subtype viruses and type B viruses contained in the vaccine. 2013 INDICATIONS AND USAGE FLULAVAL QUADRIVALENT is a vaccine indicated for active immunization for the prevention of disease caused by. 11413 US Package Insert 072018 Revision 2 Page 3 of 17 FULL PRESCRIBING INFORMATION 1 INDICATIONS AND USAGE FLUCELVAX QUADRIVALENT is an inactivated vaccine indicated for active.
Mercury from thimerosal μ g 05mL. GDS07WHO Insert02 Date of issue. The vaccination locations may provide you with a dumbed-down excerpt of the vaccine package insert.
FLUAD QUADRIVALENT - Seqirus Inc. WHO Package Insert Version number. 84 FLULAVAL QUADRIVALENT in Adults 85 Trial 1 was a randomized double-blind active-controlled safety and immunogenicity trial.
Package Insert - FluLaval Quadrivalent. In 86 this trial subjects received FLULAVAL QUADRIVALENT N 1272 or one of two 87 formulations of a comparator trivalent influenza vaccine FLULAVAL. FLUARIX QUADRIVALENT is a vaccine indicated for active immunization for the prevention of disease caused by influenza A subtype viruses and type B viruses contained in the vaccine.
WHO Package Leaflet November 2007 1. ID Biomedical Corporation of Quebec dba. February 6 2020 Approval Letter - FluLaval.
9072013 2013 GlaxoSmithKline Group of Companies Manufacturer. FLULAVAL is approved for use in persons 6 months of age and older. FLULAVAL TETRA 2018-2019 GlaxoSmithKline Approved.
5-mL multi-dose vials containing 10 doses each dose is 05 mL. US Package Insert 03 November 2020 Confidential Page 3 of 12 4 CONTRAINDICATIONS Do not administer FLUAD QUADRIVALENT to anyone with a history of severe allergic. Influenza vaccines United States 202021 influenza season.
2006 -----INDICATIONS AND USAGE----- FLULAVAL is an inactivated influenza virus vaccine indicated for. FluLaval is a trademark of the GlaxoSmithKline group of companies. NAME OF THE MEDICINAL PRODUCT FluLavalŽ suspension for injection Influenza vaccine split virion inactivated 2.
April 13 2018 Page 1 of 25 PRODUCT MONOGRAPH FLULAVAL TETRA 2018-2019 Quadrivalent Influenza Vaccine Split Virion Inactivated Suspension for Injection ATC Code. 33 FLULAVAL is a suspension for injection available in 05-mL prefilled TIP-LOK syringes and 34. Du Parc Technologique Sainte-Foy Quebec Canada G1P 4R8.
FLULAVAL QUADRIVALENT has been standardized according to US. 21 April 2020 Page 4 of 27 INDICATIONS AND CLINICAL USE FLULAVAL TETRA is a quadrivalent vaccine indicated for active immunization of adults and children from 6 months of age for the prevention of influenza disease caused by influenza virus types A and B contained in the vaccine. July 2 2020 Approval Letter - FluLaval.
HA IIVs and RIV4 or virus count LAIV4 for each vaccine virus per dose Route. Ive also included the material safety data sheets for some common additives such as Triton X-100 a severe carcinogen. GlaxoSmithKline Vaccines 2323 Boul.
ID Biomedical Corporation of Quebec Quebec Quebec Canada Distributed by. FLULAVAL TETRA 2020-2021 GlaxoSmithKline Approved. See full prescribing information for FLULAVAL.
FLULAVAL QUADRIVALENT Influenza Vaccine injectable suspension for intramuscular use 2020-2021 Formula Initial US. Package Insert - FluLaval. Here Ive gathered all the 2011-2012 flu vaccine manufacturer inserts and posted the pdf files at the bottom of this post.
FLULAVAL safely and effectively. FLUCELVAX QUADRIVALENT - Seqirus Inc. FLULAVAL Influenza Virus Vaccine Suspension for Intramuscular Injection.
 Flulaval Vaccine Guide National Vaccine Support Group
Flulaval Vaccine Guide National Vaccine Support Group
Https Www Amlc Army Mil Portals 73 Documents Flulaval 20quadrivalent 20msds Pdf Ver 2020 03 04 145821 300
 Where Can I Find Information On Flu Vaccine Side Effects I Feel Tired A Week Later And Just Want To Know If Such A Late Onset Is Possible Online Sources Are So
Where Can I Find Information On Flu Vaccine Side Effects I Feel Tired A Week Later And Just Want To Know If Such A Late Onset Is Possible Online Sources Are So
 Mercury In Flu Vaccine What To Serve A Goddess When She Comes For Dinner
Mercury In Flu Vaccine What To Serve A Goddess When She Comes For Dinner
Http Www Who Int Immunization Standards Vaccine Quality Flulaval Gsk Package Insert Text Pdf
Https Www Henryschein Com Us En Images Medical Gsk Flulaval Pdf
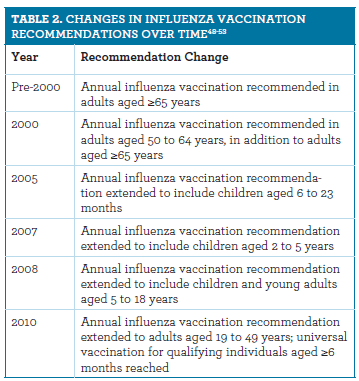 Influenza Vaccination Formulations Recommendations And Resources For The Pharmacist
Influenza Vaccination Formulations Recommendations And Resources For The Pharmacist
Https Ca Gsk Com Media 590283 Flulaval Tetra Pdf
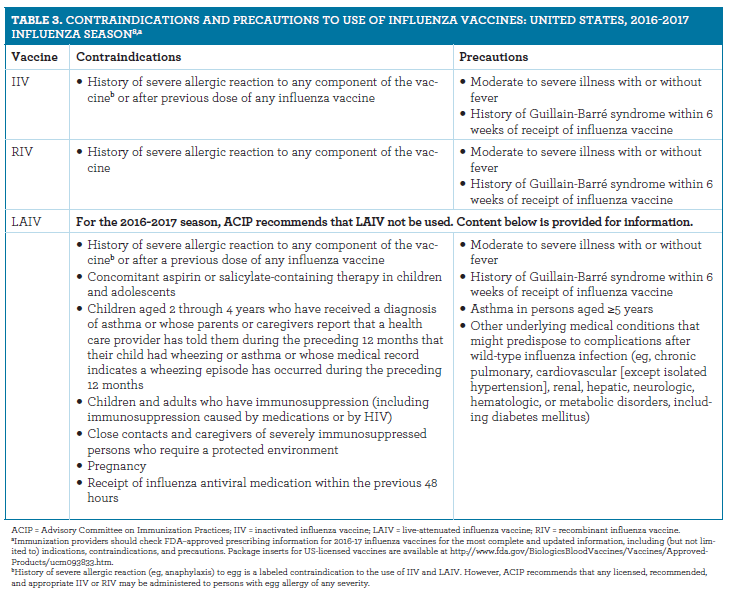 Influenza Vaccination Formulations Recommendations And Resources For The Pharmacist
Influenza Vaccination Formulations Recommendations And Resources For The Pharmacist




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.