5 it could be said that mercaptoacetic acid additions do not influence the anodic reaction order. The metal aluminium dissolves in hydrochloric acid producing aluminum chloride and colorless hydrogen gas.
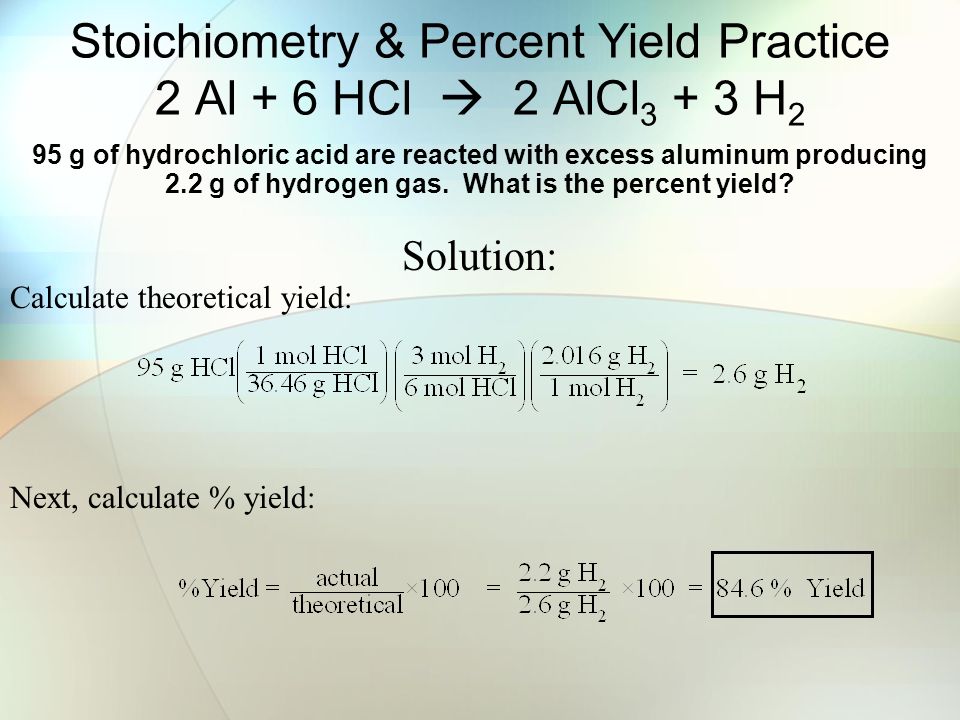 Stoichiometry Percent Yield Practice 2 Al 6 Hcl 2 Alcl H 2 95 G Of Hydrochloric Acid Are Reacted With Excess Aluminum Producing 2 2 G Of Hydrogen Ppt Download
Stoichiometry Percent Yield Practice 2 Al 6 Hcl 2 Alcl H 2 95 G Of Hydrochloric Acid Are Reacted With Excess Aluminum Producing 2 2 G Of Hydrogen Ppt Download
When aluminum reacts with a strong acid such as hydrochloric acid hydrogen gas is formed.

Aluminum hydrochloric acid. Al⁰ - 3e Al³ Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen. Aluminum Chloride and Hydrogen gas were produced in the processWow. From the result Fig.
Chat Now Send Inquiry reaction prediction. The reaction between metallic aluminum and hydrochloric acid is what is known as an oxidation-reduction reaction. Hydrochloric acid leaching is a good way to extract aluminum from secondary aluminum dross SAD.
Thus a luminium being more reactive than hydrogen displaces the latter in hydrochloric acid H C l to form aluminium chloride A l C l 3 and releases H 2 gas 2 A l. 2Al6HCl Al2Cl63H2 2 A l 6 H C l A l 2 C l 6 3 H 2. As aluminium has three electrons in its outer shell the reaction requires a ratio of two aluminium molecules to six hydrochloric acid molecules.
The balanced equation is 2Al s 6HCl aq ----- 2AlCl3 aq 3H2 g. Aluminum reacts with hydrochloric acid to form aluminum chloride. This experiment explores the reaction by measuring the volume o.
125ml Of Hydrochloric Acid Muriatic Acid 25 was added to aluminum. Calculate the mass of hydrogen formed when 25 grams 25 g r a m s of aluminum reacts with excess hydrochloric acid. However the leaching kinetics of this process is still unclear.
Aluminium reacts with dilute hydrochloric acid to give aluminum chloride and hydrogen gas. This is because each chlorine atom in the hydrochloric acid acquires an electron from the aluminium and loses a hydrogen atom. Yes acid metal -.
The balanced chemical equation for this. The reaction taking place between aluminium and hydrochloric acid is irreversible. Answer 1 of Aluminum is a poor metal and hydrochloric acid is an acid.
Aluminum reacts with hydrochloric acid to produce aluminum chloride and hydrogen gas. The balanced chemical equation that describes this single replacement reaction looks like this. Aluminum acts as the reducing agent giving up electrons.
Metal salt hydrogen What is the equation for aluminum plus hydrochloric acid. The chemical formulas for aluminum and hydrochloric acid are Al and HCl respectively. When a piece of aluminium metal is added to dilute hydrochloric acid the result is two products -.
What happens when aluminium reacts with hydrochloric acid. Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride AlCl3 and hydrogen gas H2. Keep in mind that this reaction will not take place as soon as you add the piece of aluminium to the hydrochloric acid solution.
5 reflect the reaction order with respect to aluminium alloy ie the anodic reaction. The reaction between aluminium and hydrochloric acid. Aluminum reacts with diluted hydrochloric acid at room temperature.
2Al s 6HCl aq - 2AlCl3 aq 3H2 g What mass of H2 g is required from. Hydrogen gas is also formed during this reaction. The anodic dissolution reaction of aluminium in hydrochloric acid solution is as follows.
Aluminium plus hydrochloric acid yield aluminium chloride plus hydrogen gas. Hydrochloric Acid Reacting With Aluminum - Duration. A number of aluminum-containing raw materials can be used including aluminum metal alumina trihydrate aluminum chloride aluminum sulfate and combinations of these.
Aluminum reacts with hydrochloric acid in a single-displacement reaction resulting in aqueous aluminum chloride a salt and hydrogen gas. Aluminum reacts with hydrochloric acid to produce aluminum chloride and hydrogen gas. Aluminum chlorohydrate can be commercially manufactured by reacting aluminum with hydrochloric acid.
