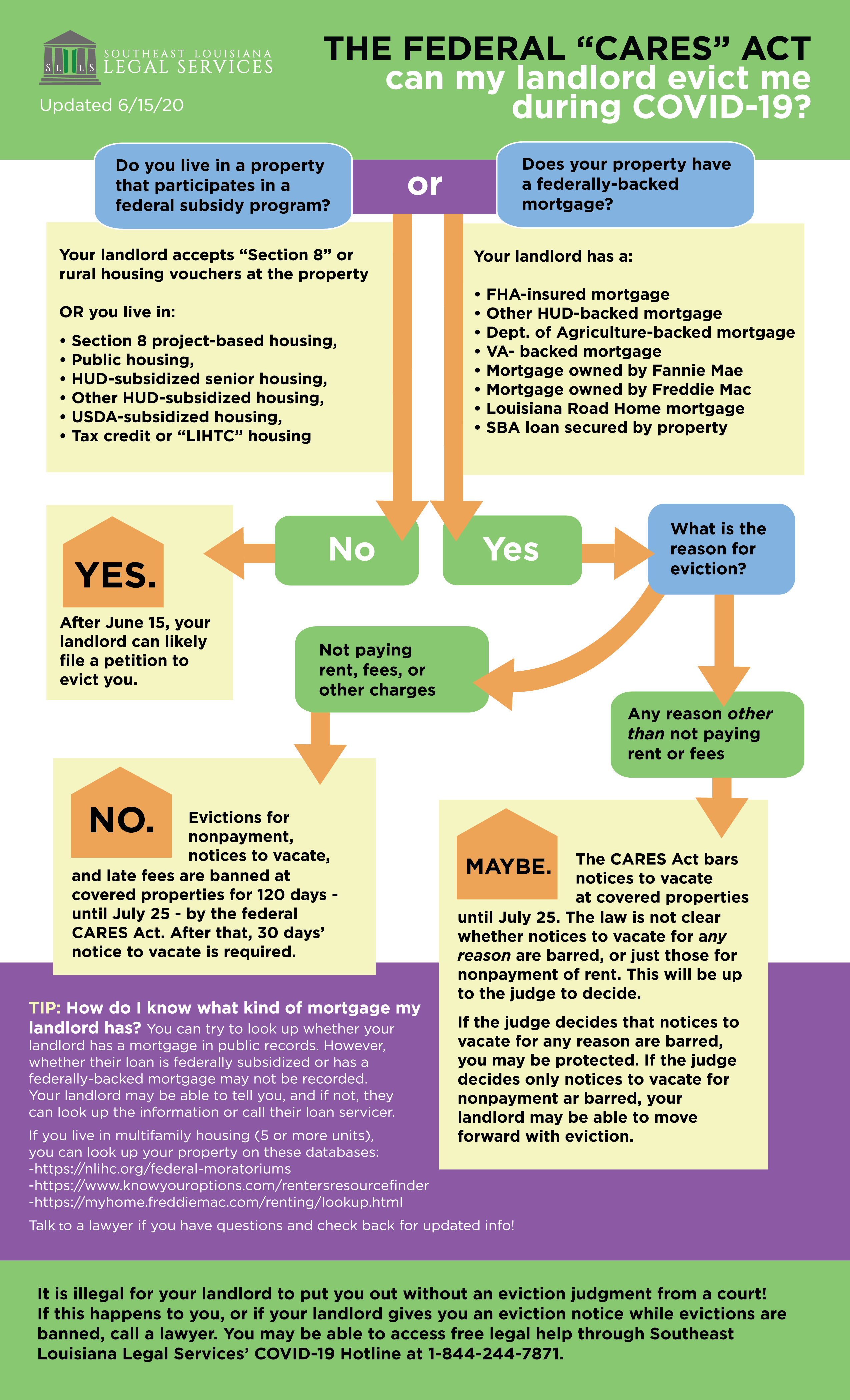
Current supplies of publicly funded Zostavax II LZV expire in October 2020. To avoid causing an injury do not inject too high near the acromion process or too low.
 What To Do If Shingrix Is Accidentally Given Sq Instead Of Im
What To Do If Shingrix Is Accidentally Given Sq Instead Of Im
The use of Shingrix should be in accordance with official recommendations.

Shingrix im or sq. Shingrix is recommended for the prevention of herpes zoster and related complications for immunocompetent adults who previously received Zostavax. Give in the central and thickest portion of the deltoid. Adults 50 years of age or older.
After you have been infected with chickenpoxusually in. Zoster is caused by the same virus that causes chickenpox. Use anterolateral thigh muscle 58 needle 2225 gauge Note.
Shingrix recombinant zoster vaccine. Shingrix Zoster Vaccine Recombinant Adjuvanted is a sterile suspension for intramuscular injection. Shingrix is publicly funded for 2 doses in adults ages 65 to 70 years who have not previously received the publicly funded LZV.
Adults 18 years of age or older at increased risk of HZ. However it is not necessary to repeat vaccination if it is administered intramuscularly. Use in pregnancy Pregnancy Category B2 There are no data on the use of SHINGRIX in pregnant women.
The CDC now recommends RZV as the preferential shingles prevention vaccine. Shingrix is recommended for the prevention of herpes zoster and related complications for immunocompetent adults 50 years of age and older. The RZV vaccine was approved October 2017 for adults aged 50 years and older for the prevention of herpes zoster shingles.
Muscle above the level of the armpit and approximately 23 fingerbreadths 2 below the acromion process. It is administered as a 2-dose intramuscular IM series with the second dose given anytime from 2 to 6 months after the first. The recombinant zoster vaccine brand name Shingrix is a two-dose vaccine that is more than 90 protective against shingles even among the elderly.
This infographic promotes safe vaccination practices by educating and reminding providers about proper intramuscular IM vaccine administration technique for Shingrix. Shingrix is the preferred vaccine over Zostavax. Injection site and needle size For newborns 028 days.
Shingrix the recommended vaccine for shingles prevention is indicated to be given by IM intramuscular injection in the deltoid. Adults 50 years of age or older. Adults 18 years of age or older at increased risk of HZ.
For neonates first 28 days of life and preterm infants a 58 needle is recommended if the skin is stretched flat between the thumb and forefinger and the needle is inserted at a 90-degree angle to the skin. It helps prevent shingles herpes zoster in adults ages 50 and older. SHINGRIX 01 and 02 mL respectively by IM injection on 42 28 and 14 days prior to mating male and 28 and 14 days prior to mating female had no effects on mating or fertility.
Mercks Zostavax is a subcutaneous one-time injection while Shingrix is an intramuscular IM injection requiring 2 injections with the second dose given 2 to 6 months after the first. Intramuscular IM injections. Shingrix is a brand-name vaccine.
The vaccine should not be injected intramuscularly. Shingrix is indicated for preventi on of herpes zoster HZ and post-herpetic neuralgia PHN in. Administering Shingrix Shingrix should be injected intramuscularly in the deltoid region of the upper arm.
The vaccine is supplied as a vial of lyophilized recombinant varicella zoster virus surface glycoprotein E VZV gE which is reconstituted at the time of use with the accompanying vial of AS01B adjuvant suspension. This vaccineis used by adults 50 years and older to prevent zostershingles. Beginning mid-October Ontarios publicly funded shingles program will change vaccine to Shingrix.
However if there is an administration error and it is accidentally given SQ subcutaneously the CDC Centers for Disease Control states that. Pain due to injection was reported in roughly 40 of patients receiving Zostavax compared to 78 with Shingrix as expected with an IM injection. INDICATIONS AND CLINICAL USE.
RZV is a new GlaxoSmithKline shingles vaccine that was just placed on the market in October 2017. The Shingrix vaccine isnt approved for use in adults younger than age 50. Zostavax zoster vaccine live is administered subcutaneously as a single dose in the deltoid region.
42 Posology and method of administration. Shingrix is indicated for prevention of herpes zoster HZ and post-herpetic neuralgia PHN in. The use of Shingrix should be in accordance with official recommendations.
SHINGRIX is a sterile non-live vaccine for intramuscular injection. Route subcutaneous intramuscular. The vaccine is supplied as a vial of lyophilized recombinant varicella zoster virus surface glycoprotein E gE antigen component which must be reconstituted at the time of use with the accompanying vial of AS01 B adjuvant suspension component.